The National Agency for Food and Drug Administration and Control (NAFDAC) has issued critical public health alerts concerning counterfeit and recalled pharmaceutical products. These alerts, shared on the agency’s official X (formerly Twitter) page on January 2, 2025, aim to safeguard public health by informing healthcare providers, distributors, and consumers about the potential dangers of these products.
Below are the detailed updates from the alerts:
1.Public Alert No. 051/2024.
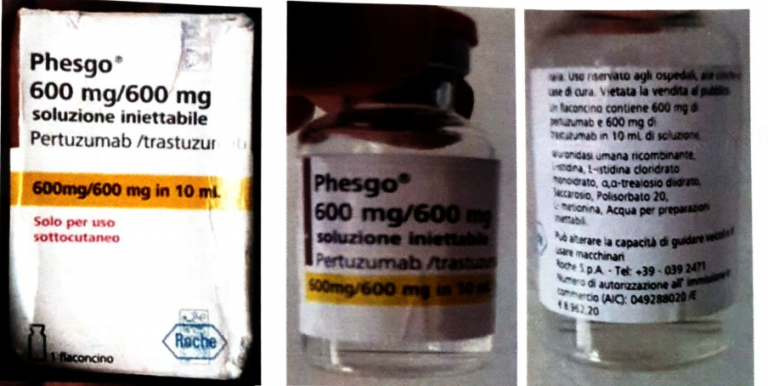
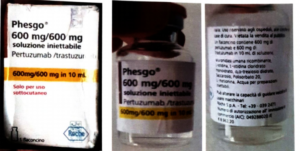
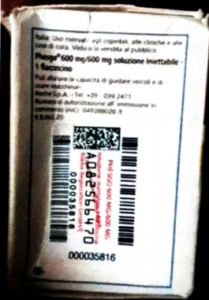
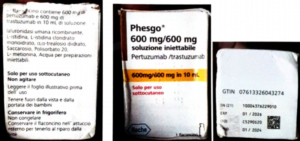
Alert on confirmed counterfeit PHESGO 600mg/600mg/10ml With batch number C5290S20 stated to be manufactured by Roche S.p.A
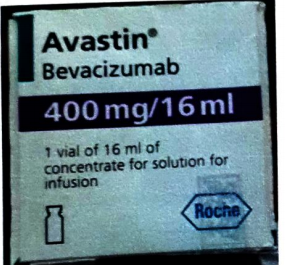
2.Public Alert No. 052/2024.
Alert of confirmed cases of two counterfeit batches of Avastin 400mg/16ml, manufactured by Roche Diagnostics Public Gmbh, Mannheim, Germany.

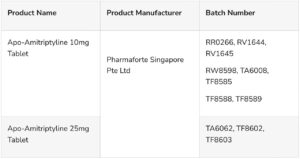
3. Public Alert No. 050/2024.
Alert on the recall of Apo-Amitriptyline 10mg and 25mg manufactured by Pharma Forte Singapore Pte Ltd.
Counterfeit and substandard drugs pose significant health risks, including therapeutic failure, adverse reactions, and exacerbation of health conditions. As part of its mandate to safeguard public health, NAFDAC continues to monitor, detect, and combat the circulation of harmful pharmaceutical products in Nigeria.

For additional information, visit NAFDAC’s website at http://www.nafdac.gov.ng or call their hotline.
...Never miss an update again! Join our WhatsApp group for the latest news, straight to your phone! (Click Here)